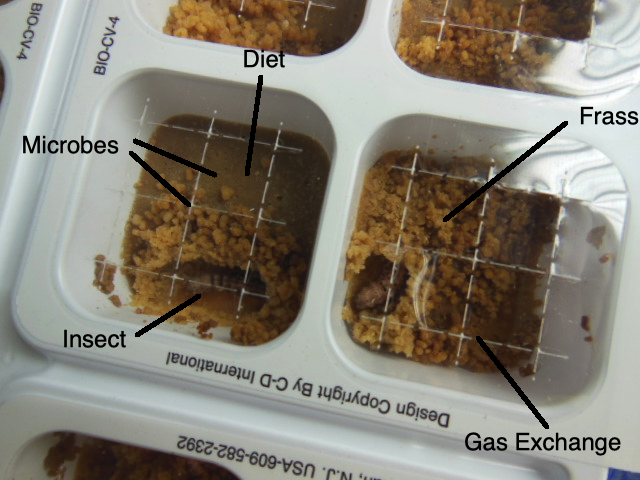
A central tenet of the rearing program at NCSU is that insect rearing systems are artificial ecological niches of the insects we rear. This means that every aspect of the insect’s needs must be met by our rearing system. This responsibility of rearing personnel applies whether we know each requirement or not. For example, each insect (and each insect population in our colonies) requires a certain range of oxygen to meet their metabolic needs. We may not know how much oxygen this requires (the range of oxygen concentrations), but when we provide holes in the lid of the rearing container or a screen that permits gas exchange, we hope that the openings are adequate to meet the oxygen demands.
Therefore, we can think of the oxygen requirements as part of our insects’ ecological niche, and if our insects perform adequately under the conditions of our rearing containers, we assume that everything is OK “oxygen-wise.” We make the same kinds of assumptions about carbon dioxide concentrations and air flow and the same about water vapour. Sometimes we get ourselves in trouble when we try to restrict water loss from he diet or the insects by making the openings so limited that we wind up starving our insects of adequate oxygen (a phenomenon known as hypoxia or even anoxia); and/or we make create a situation of excess carbon dioxide, which can threaten our insects’ well-being.
In all the rearing courses that I teach, I try to convey to students that the rearing system with all its components that meet the insect’s ecological niche parameters is a complex set of interactions between various (ALL) components, and a helpful way of viewing all this is with a 3-D model or diagram such as what is seen here:
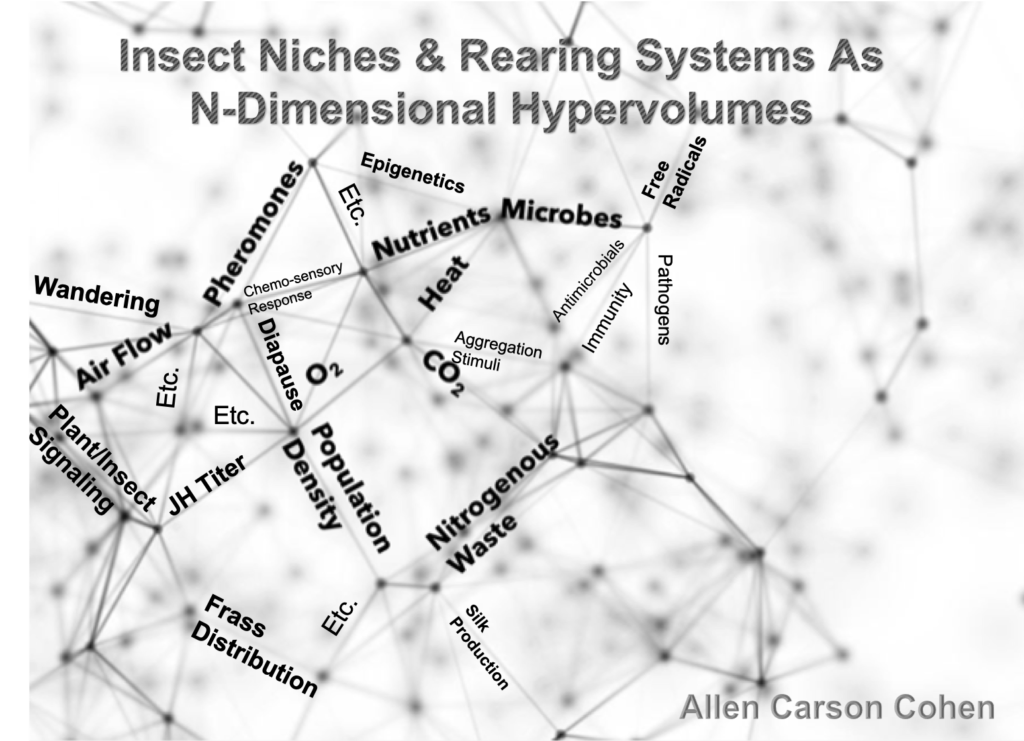
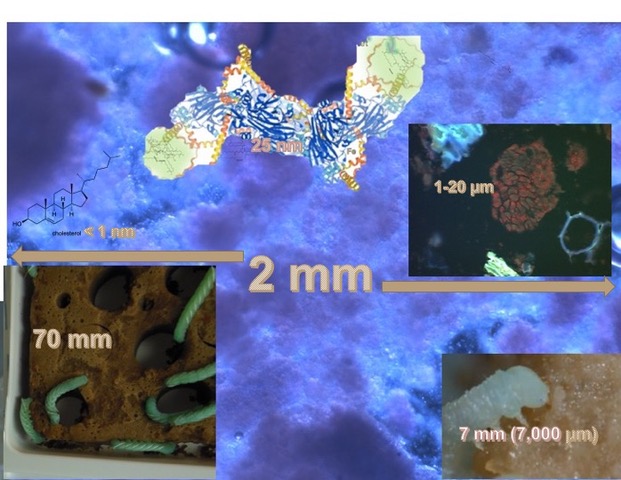
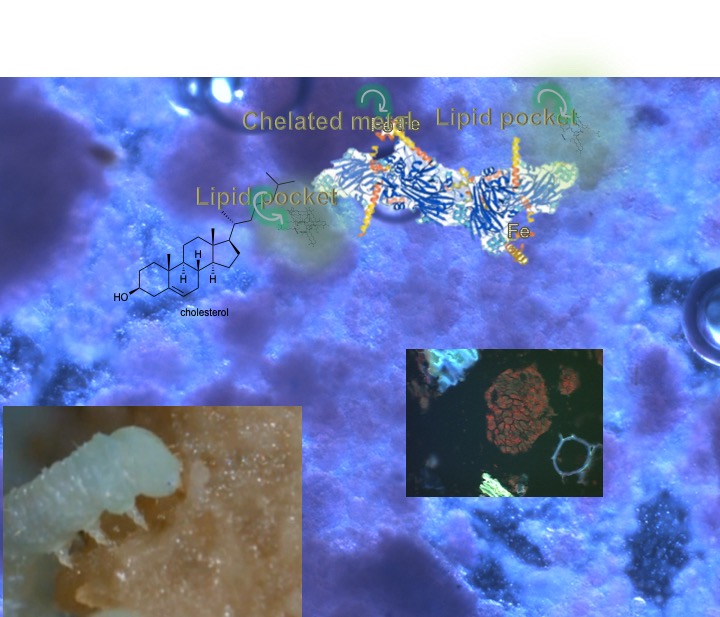
In this diagram that simulates or suggests three-dimensional space, I have tried to show about 20 of the many, many parameters in a niche. The diagram is intended to illustrate that there are interconnections and interactions that involve all the biotic (biological) and abiotic (physical/chemical) factors that play roles in our insects’ well-being. This model of N-dimensional hyperspace is derived from the work of the famous ecologist G. Evelyn Hutchison. I have used this model in my recent book, Design, Operation, and Control of Insect Rearing Systems 2021, CRC Press, Boca Raton, FL).
For this discussion, let us take-up a simplification of this diagram with 3 factors, heat, CO2, and O2. Here is the simplified diagram:

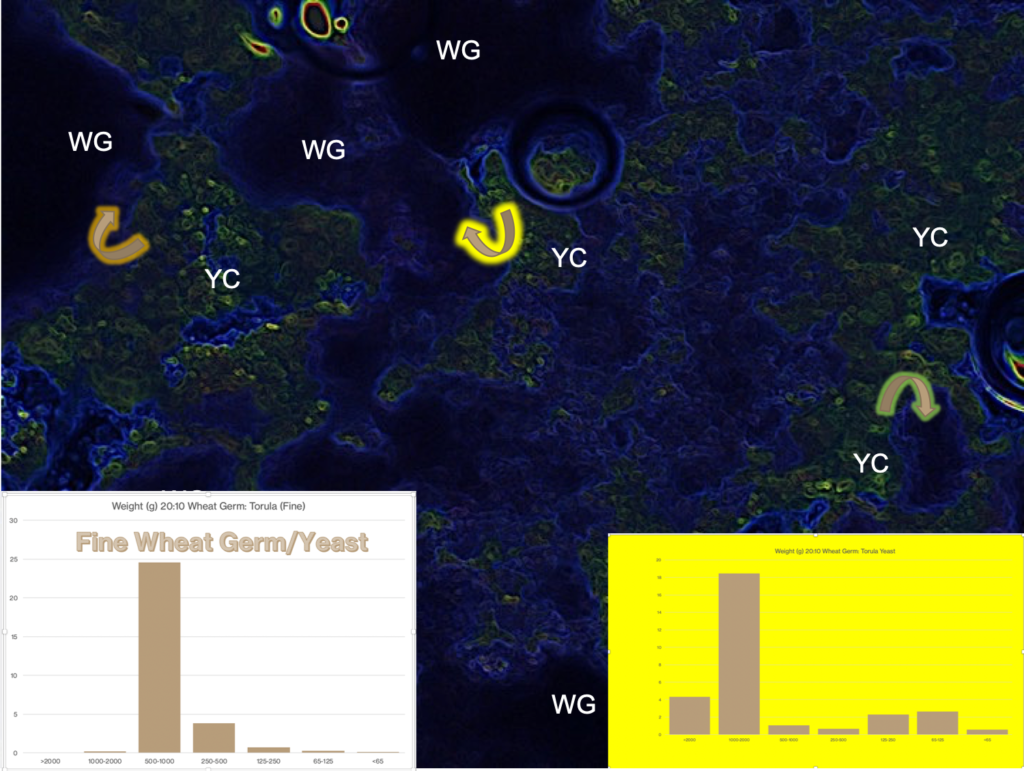
In this 3-D, three-factor diagram, I am trying to show that the waxworm from my colony (of Galleria mellonella) is greatly influenced by the heat (temperature) conditions in their rearing container; but also, the concentration of CO2 and O2 are interacting factors that influence the metabolism and aggregation behavior of the waxworms, as well as food consumption, digestion rates, development rates, etc.
These interactions are illustrated in the images taken from my waxworm colony where I used a thermal imaging camera to capture the heat (thermal) gradient produced by the waxworms in this container.
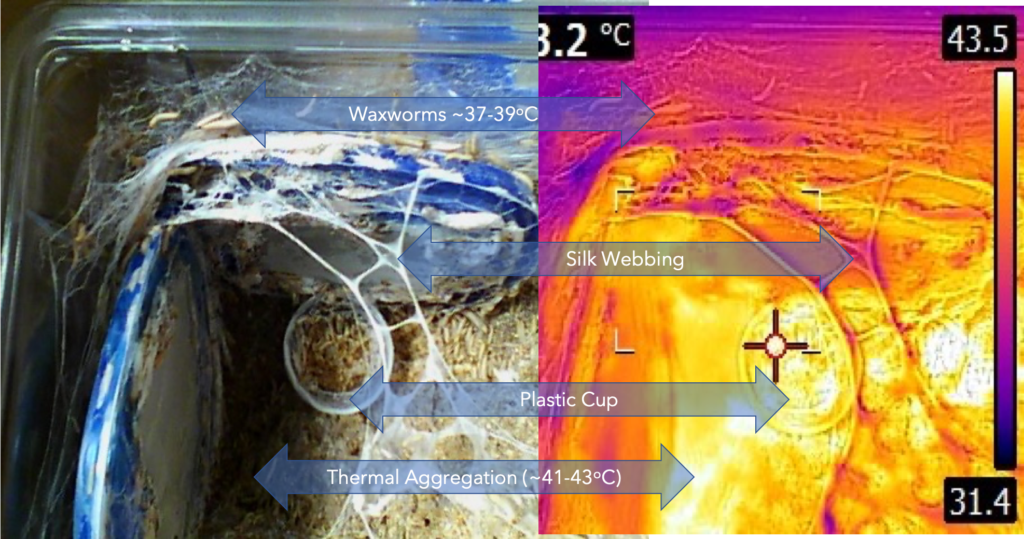
In the study of the colony described here, I have also included (besides the thermal profile) the CO2 and O2 gradient that results from the waxworms’ behavior and metabolism. The example, taken from waxworm cultivation in my research program, is typical of the kinds of multi-dimensional dynamics that apply to ALL insect rearing systems. My whole point in this and related discussions is to get students of insect rearing to recognise the complexities of their rearing systems components. It is further intended to awaken students’ appreciation for knowing the nature of (the science of) these many factors and their interactions.
This approach is not simple or easy; but it is very powerful in establishing and maintaining healthy, productive colonies!